Background
Although many reports have established the efficacy of post-transplantation cyclophosphamide (PTCy) or ATG for GVHD prophylaxis to in vitro non-T cell-depleted (non-TCD) haplo-HSCT and achieved a good clinical effect, the overall results of NRM, RI, DFS, and OS still need to be improved, especially for patients with high-risk or R/R hematologic malignancies. We speculated that if GVHD could be controlled well, higher doses of in vitro non-TCD peripheral blood stem cells (PBSCs) as a graft source for haplo-HSCT may be able to better balance engraftment, immune reconstitution, infection, and graft versus leukemia (GVL) effect to improve clinical outcomes. Apparently, due to the increased risk of GVHD as a major concern, the research of haplo-HSCT using ultra-high doses of in vitro non-TCD PBSCs as a graft source is now scarce. To this end, we constructed a unique haploidentical high-dose peripheral stem cell transplantation (haplo-HDPSCT) protocol. A prospective study was performed in our center using this protocol to treat patients with hematologic malignancies.
Methods
From January 2006 to December 2018, a total of 346 patients with hematologic malignancies less than 50 years old were enrolled. 205 patients who had no matched sibling donor (MSD) enrolled in the haplo-HDPSCT group, and 141 patients were enrolled in the MSD-PSCT group. Patients in two groups were comparable except for several characteristics such as more young and high-risk patients in the haplo-HDPSCT group. All patients were followed up until December 2022 with a median follow-up time of 66.1 months. The MSD-PSCT protocol was performed with the classical standard process. Our haplo-HDPSCT protocol was designed adopting high-dose in vitro non-TCD PBSCs as graft, Ara-C+Bu/Cy+rATG as the conditioning regimen, and Basiliximab plus short-term low-dose GCs added to standard GVHD prophylaxis. The long-term outcomes of the two groups were analyzed and compared. The primary endpoint was aGVHD, and the second endpoints were engraftment, infection, RI, DFS, and OS.
Results
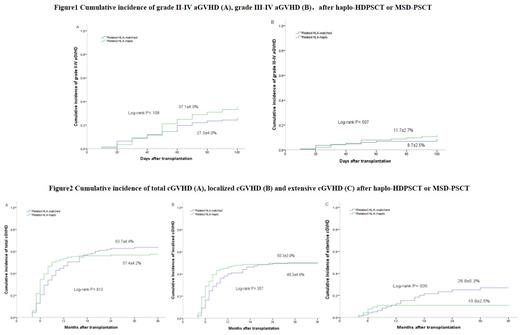
In the haplo-HDPSCT group(n=205), the infused median mononuclear cells(MNCs), CD34+ cells, and CD3+ cells were 15.63×10 8/kg, 8.59×10 6/kg and 5.39×10 8/kg, respectively, which significantly higher than those in the MSD-PSCT group(n=141). Only 3 of 205 patients in the haplo-HDPSCT group developed primary graft failure (PGF) and the primary engraftment rate was 98.5%. The incidence of grades II-IV and III-IV aGVHD within 100 days was similar between the two groups (37.1% vs. 27.3%, P=0.109; and 11.7% vs. 8.7%, P=0.507). The 3-year cumulative incidence of localized cGVHD was similar between the two groups (50.3% vs 49.3%, P=0.357), but the extensive cGVHD in the haplo-HDPSCT group was significantly lower than that in the MSD-PSCT group (10.9% vs 26.9%, P=0.035). The 3-year cumulative RI and NRM were also similar between the two groups (RI: 31.1%vs 27.3%, P=0.625; NRM: 16.1% vs 11.5%, P=0.394). No significant differences in 3- and 5-year OS (69.6% and 66.9% vs. 74.9% and 73.2%, P=0.282) or 3- and 5-year DFS (67.2% and 63.7% vs. 72.0% and 69.4%, P=0.215) were observed in the haplo-HDPSCT group and the MSD-PSCT group. The CMV disease occurrence was 3.1% in the haplo-HDPSCT group. The EBV-DNAemia rate was 10.4% in the haplo-HDPSCT group and 5.7% in the MSD-PSCT group(P=0.836). Only two patients in the haplo-HDPSCT group developed PTLD.
Conclusions
This study demonstrates that aGVHD is not high in our haplo-HDPSCT protocol and the long-term follow-up shows that this protocol may bring benefits including high engraftment rate, less relapse and viral infection, which implies that higher doses of in vitro non-TCD PBSCs as a graft source for haplo-HSCT protocol is feasible if appropriate GVHD prophylaxis is adopted.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal